|
Was Darwin wrong? The sole descent of the domestic pigeon from the
rock pigeon
Molecular genetic studies also put many beliefs in pigeon breeding
to the test. An important finding of recent studies on the check
pattern in domestic pigeons and on the Stipper gene and its alleles
was that colorations were associated with a structural change in the
genetic make-up by changing the number of copies of segments of DNA
(Copy Number Variation CNV) in a genome (Vickrey et al. 2018,
Bruders et al. 2020). Not every deviation from the wild type is due
to mutative changes in the base sequences in a DNA segment. Based on
research in human genetics after 2000, this has already been
investigated for other animal species. The findings also show much
in pigeon breeding that was previously believed in a new light.
Another thesis derived in connection with the study of the pattern
is more provocative. Namely, that the genes for the check pattern
were very likely transferred from the Guinea pigeon to the domestic
pigeon. In a period of time only centuries ago, and far after the
species separated millions of years ago.
Darwin and the pigeons
For Darwin, the various breeds of domestic pigeons all come from the
rock pigeon (C. livia), of which he had a drawing of a shot
individual in his work 'The variation of animals and plant under
domestication' (1868, 2nd ed. 1875). The wild and semi-wild pigeons
in the English 'Dovecots' were a typical link in domestication. The
occasional and non-unique appearance of checks in otherwise blue-bar
wild and semi-wild populations was a domestication phenomenon for
him (1868, 1875). The deviation of the pattern was no justification
to consider checks as a subspecies in the rank of the somewhat
darker rock pigeon variant in the Indian region or the somewhat
lighter variant in the Nile region. In some regions of England,
half-wild check pattern pigeons were so common that Dixon described
them as a typical 'Dovecote-Pigeon' in his book in 1851 and depicted
an individual. For a discussion of the check variant, which has
meanwhile been named by Blyth C. affinis, see Darwin 1875 Vol. 1
Chapter VI.
 
Fig. 1: The Rock Pigeon at Darwin 1868/1875 and the blue check
‚Dovecot-Pigeon‘ (Columba affinis at Blyth). Source: Dixon 1851
Checkered pigeons before Darwin
Going back further in time, Albin has a drawing of a check 'Dovecot
pigeon', dated 1735, with reference to the wide range of variation
of these half-wild pigeons at the time. This can already be seen in
the coloration of the individual shown and in the text, when
referring to the check, 'black with ash-gray admixture' (mixture
with cinereous) is written. From today's point of view artistic
freedom or even a diluted check and an early hint on a gene later
named dilution.

Fig. 2: The Common Dove House, or Wild Pigeon, dated 1735. Source:
Albin, Natural History of Birds 1738 Vol III (sheet 39)
In Thuringia, Bechstein, who was not only a scientist but also a
pigeon keeper, described on domestic semi-wild stocks how new colors
were created in the process of domestication (1795, 1807). Among
other colors, the 'gedüpfelte' = checkered field pigeon.
 |
Since I am a great friend of these birds, not only as a
naturalist, but also as a fancier, I have taken great
care to observe how the various tame varieties of this
wild breed gradually
form the various tame varieties of this wild race
(because they still belong among them).
From this common wild pigeon, the checkered field pigeon
described below first emerges, even if it is not fed in
the house. This gradually becomes reddish-gray and
pearl-gray with red-brown strings, fox-red and very dark
blue; then the wings and tails
vary, initially becoming light gray ...
|
Fig. 3: Domestication and the variation in coloration, Bechstein
(1795), p. 18.
About 200 years earlier, Marcus zum Lamm had field pigeons (ferrals
or in an old German terminology 'Feldratzen') painted around 1600,
one of the pigeons undoubtedly in the check pattern. In his notes he
writes about checkered pigeons (a Visch Schüppichte or hammerschlegichte Daub), quoted in Kintzenbach / Hölzinger 2000, p.
189.

Fig. 4: Field pigeons or Feldt Ratzen at Marcus zum Lamm around 1600
(Kintzenbach/Hölzinger 2000)
In the falcon book 'De arte venandi cum avibus', written by
Friedrich II between 1259 and 1266 and illustrated also with
drawings of pigeons. Not only falcons are discussed but the work is
considered an early ornithology using earlier sources besides own
observations. If the pigeon depicted individually is supposed to
represent the bluebar archetype (fol. 18), then the domestic pigeons
shown together with wild pigeons (fol. 11) should also be checkered
deviations from the bar pattern. Interesting is an even earlier
illustration from the time before Christie's birth, printed in the
book by Daniel Haag-Wackernagel, which is interpreted as check.
  
Fig. 5: A blue house pigeon and house pigeons together with wild
pigeons in the falcon book Friedrich II 1241-1248, fol. 18 and fol.
11, wall painting from the green room of the north palace of Tell el
Armana, Egypt 1350 BC Chr. (Printed in Haag-Wackernagel 1989, Sell
2009)
Evolution of the pigeon species
For some groups of pigeon species, Goodwin has tried to trace when
individual groups and species, starting from a common origin, split
up and split off.
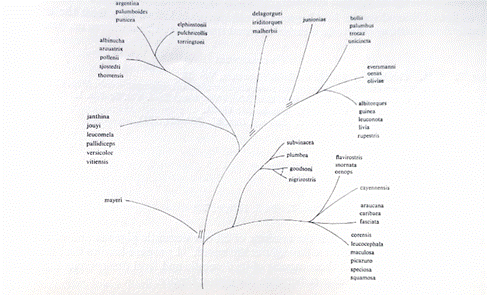
Fig. 6.: Assumed relationships in the Columba genus in Goodwin,
Pigeons and Doves of the World, second ed. London 1970, p. 53.
The rock pigeon, and the domestic pigeon derived from it, is
regarded as C. livia as being closely related to the amphara pigeon,
the snow pigeon, the guinea pigeon and the cliff pigeon or Eastern
rock pigeon. This branch is shown separately here.
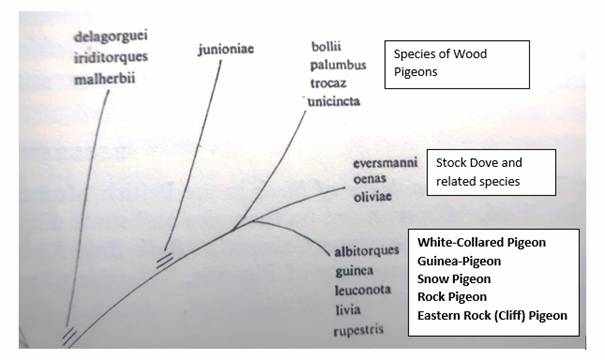
Fig. 7: Excerpt from the diagram on the relationships in the Columba
genus in Goodwin, Pigeons and Doves of the World, second ed. London
1970, p. 53 with text addition
The wild forms of pigeon species whose paths separated earlier in
their developmental path are more different in appearance and
behavior from one another than species in which this happened later.
According to the diagram, the white-collared (amphara) pigeon,
guinea pigeon, snow pigeon and cliff pigeon have followed a common
development path longer than with the stock pigeon and the wood
pigeon that had split off before. The longer in the past the split,
the greater the likelihood that behavior and appearance of the wild
form will also develop differently due to different mutations in the
separate groups and due to the loss of previously common genes in a
population. The genetic similarity of species and the assumed
periods of time are shown in a molecular genetic study with the
attempt to classify closely related species by Soares et al. 2016.
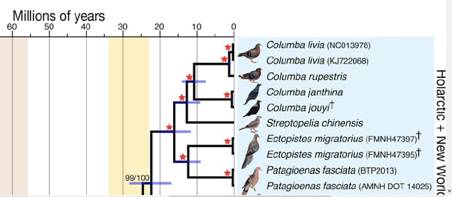
Fig. 8: Clustering of wild pigeons according to genetic similarity
by Soares et al. 2016 (excerpt)
Species that are already extinct are also included in the study. The
guinea pigeon was not there, nor the stock dove and the wood pigeon.
The two examples of the Columba livia are, according to the sources,
to presume Gimpel pigeons as a domestic pigeon breed and rock
pigeons with little genetic distance to it. The closest related
species is the cliff pigeon C. rupestria, which outwardly hardly
differs from the C. livia except for the typical white lightening in
front of the tail band. In amateur breeding, the trait is anchored
in Bern mirror tails. The bleaching of the tail feathers in both
cases differs from the darker-framed mirrors of oriental owls.
Similar bleaching occurs in combination with other hereditary
factors in a weakened form in other domestic pigeon breeds (see also
pictures in Pigeon Genetics 2012 and Genetics of Pigeon Colorings
2015), possibly a shared inheritance of a common preform or an early
introgression.
Introgression and 'retrogression' from a historical perspective
Introgression, in the title of the 2018 study cited, means the
transfer of genes from one species to another after the formation
and consolidation of different species. According to the model
calculations, the period of the transfer of the check pattern from
C. guinea to C. livia is estimated to be 429 to 857 years ago with a
generation succession of one to two generations per year (Vickrey et
al. 2018, p. 12). In retrospect, that would be around AD 1200 -
1600. It is the time when Friedrich II, Emperor of the Roman-German
Empire, wrote the falcon book 'De arte venandi cum avibus'
(1258-1266) and when Marcus zum Lamm wrote his notes on the 'Picturarum'
and had pictures of domestic pigeons and field pigeons painted
(around 1600). The centuries in between were marked in Europe by the
plague in the 14th century and by armed conflicts. At the end of the
15th century, America was discovered by Europeans and after 1600 the
Thirty Years War devastated many parts of Europe. The distribution
of the guinea pigeon should not have deviated from the distribution
given by Goodwin at the time. There has been an overlap in the
distribution of the rock pigeon and the guinea pigeon in some parts
of Africa. It is unlikely that crossbreeds with domestic pigeons
would have taken place in Europe at that time.
 
Fig. 9: Distribution maps for the rock pigeon and the guinea pigeon
at Goodwin (1970)
From historical circumstances, it was assumed that the sporadic
appearance of check variants in widely separated parts of Central
Europe was due to repetitive mutations and not to deliberate or
accidental crosses with the guinea pigeon. The check pattern is
dominant and, unlike a recessive gene, cannot be obscured in a
population for long until it appears sporadically. Bechstein's
observation (1795) of the emergence in wild-colored field pigeon
populations in Thuringia and the occurrence in populations on the
coasts of England and on the Faroe and Orkney Islands, in the far
north of the distribution area of the rock pigeon (Darwin), can
hardly be explained with crossbreeds between the arts.
A summary of the results of crossings of wild pigeon subspecies with
one another can be found in Gray 1958. The list, here an excerpt,
shows the problems of maintaining a first generation even under
controlled conditions.

Fig. 10: Bird Hybrids, excerpt from Annie P. Gray 1958, p. 128.
Successful rearing of hybrids in crossings with guinea pigeons has
nevertheless been reported several times. Dietmar Fennelt, who
brought cliff pigeons to Germany, reported on quadruple crossings
with proportions of stock pigeons, rock pigeons, guinea pigeons and
cliff pigeons, which would have resulted in a consistently fertile
line (messages in an internet blog and personal). That it was
possible to obtain offspring from the male hybrids even in infertile
female hybrids from rock pigeon x guinea pigeon and, after two
mating back of cocks to blue-bar C. livia, also fertile offspring (Vickrey
et al. 2020, p. 10 with reference to Taibel, 1949) does not have to
mean that it also takes place in the wild and that the genetic
material has been permanently incorporated into the population. In
2004 Stauber reported about an introgression from crossing Bern
mirror tails, a Swiss color pigeon, with cliff pigeons.

Fig. 11: Bern mirror tail and cliff pigeon (Source: Sell, Genetik
der Taubenfärbungen 2015 (Genetics of pigeon colors))

Fig. 12: F1 from ‚Bern mirror tail‘ and cliff pigeon
(Photo Karl Stauber, reprinted in Sell, Taubenzucht, Achim 2019)
Unexpectedly, because of the great similarity, it was initially not
possible to raise young in the first cross. The chicks seemed to
need a longer supply of pigeon ‘milk’. However, by mating the few
hybrids finally raised back to Bern mirror tails, fertile offspring
were obtained, which inherited the mirror tails of the parent
animals on both sides. To speak of introgression into the domestic
pigeon population is nevertheless an exaggeration. For one thing,
the mirror tail was already there before the intersections. The
intermediate first generation in crossbreeds rather indicates
parallel developments in this trait. Second, the Bern mirror tails
are a rare breed themselves, and breeds and lines are disappearing
with breeders. Genes that have potentially flowed in through
crossbreeding can also be lost again, resulting in 'retrogression'.
Molecular genetic evidence
For investigations into the extent and when an introgression between
two species took place, the D-statistics are used in more recent
studies. Methodologically, the procedure is similar to a cleverly
designed chi-square test. Check and bar C. livia are compared with
Guinea pigeons and, as a reference (in the terminology of the
methodology, the 'outgroup population'), the wood pigeon. Their
genome serves as a representative of the genome of the original art
before the split. All of the individual species that have emerged
from the original specie have parts of their origins in their
genome. In the further evolution, different new mutations in the
genome have prevailed in the new species split off from it over the
course of millennia and sometimes for longer periods of time. Other,
originally existing hereditary factors have been replaced. The
genetic makeup has changed. If check and bar C. livia do not
represent different arts or one of them contain external parts by
former introgression, then it is to be expected that they have the
same proportion of the genetic make-up of the 'outgroup population'
with regard to the genetic makeup.
There are sequences of chromosomes of the bar C. livia (designated
as P1), the check C. livia (P2), the C.
guinea, which also has the check pattern (P3) and, as 'outgroup
population', sequences of the wood pigeon.The null hypothesis is
that P1 and P2 are descended from a common
parent species and after the separation did not receive any gene
inflow from one of the also split off P3s. The
alternative hypothesis is that of introgression following the
separation of the species, whereby the check trait could have been
transmitted. Methodologically, we speak of the ABBA and BABA
configuration. Areas of the genome in which there is overlap in the
four populations are considered. The ABBA configuration refers to
areas in which P1 has the outgroup allele and P2
and P2 the acquired allele (derived copy). The
configuration BABA corresponds to areas in which P1 and P3
have the acquired alleles and P2 the outgroup allele
(Durand et al. P. 2240, and related thereto, Vickrey et al., 2018,
p. 10). In the formulation by Durand et al .: “For the ordered set
{P1, P2, P3, O}. we call the two
allelic configurations of interest "ABBA" or "BABA." The pattern
ABBA refers to biallelic sites where P1 has the outgroup
allele and P2 and P3 share the derived copy.
The pattern BABA corresponds to sites where P1 and P3
share the derived allele and P2 has the outgroup allele
”.
The D statistic measures empirically which configuration is more
plausible by determining the difference in the measured genetic
similarity in the ABBA and BABA configuration. This is normalized by
the sum of the two values (cf. Durand et al. P. 2240).
Maybe another kind of departure is more obvious. The similarities
are measured
P1 O + P2 P3 for a potential ABBA
figuration, and P1 P3 + P2 O for an
alternative BABA figuration. For the D statistic follows
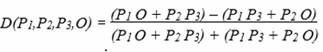
If P1 and P2 have not experienced
introgression, then the measured similarities with the other
populations will be within the scope of statistically insignificant
deviations. The similarity P1 O will correspond to the
similarity P2 O (P1 O ≈ P2 O). P2
P3 and P1 P3 will also correspond
(P2 P3 ≈ P1 P3).
Readable on the numerator, the summands in the numerator will
largely neutralize each other and the D statistics will approach
zero. Larger deviations from this can be interpreted as an indicator
of introgression.
The hypothesis was assumed to have been also confirmed in the samples with D Statistics
close to zero for a consideration of the entire genome (Vickrey
et al., Pp. 12, 17). Tests for the candidate region, in which the
traits for the pattern and also the genes for checks are located,
however, showed in contrast that the checkered C. livia was
similar to the C. guinea and not the barred C. livia (p. 12). The
hypothesis was that check C. livia should be more similar to bars here than
to C. guinea. According to the high D Statistic values, the
candidate region appears to essentially comprise the genome regions
responsible for the checks. The areas also do not seem large enough
to be mixed up to a greater extent by linkage breaks and the
exchange of factors not directly responsible for the checks. In
contrast to the domestic pigeon, the area relevant for checks in the
guinea pigeon showed no multiple copy variation (MCV) (p. 12). This
could also indicate the need for other explanations which are
indicated below.
Consideration beyond the test
Identical features as in C. livia can also be found in subspecies of
the turtle dove (genus Streptopelia) such as the domesticated
Ring-Neck Dove or Barbary dove (Streptopelia, risoria). There are
whites with dark eyes, albinos with pink eyes, silky feathered ones
and those with feather structures that differ from normal (Goodwin
1970, p. 129). Gray also reported on offspring from matings with C.
livia in 1958. James Demro raised a peak-crested young from a
peak-crested satinette pigeon with a peak crested dove (Miller/Demro
2011). Since the peak-crest is a recessive feature in the domestic
pigeon and the doves as well, first of alleles were initially spoken
of.
 
Fig. 13: Cross of Satinette (domestic pigeon) x Ring-Neck Dove with
a peak crested young. Source: Miller/Demro 2011.
Subsequent molecular genetic analysis by a research group at the
University of Utah (Vickrey et al. 2015) showed, also based on this
animal, that the mutation in the wild pigeon indeed was a recessive
also in that species, but was not located directly in the genome at
which it was suspected based on the location at the rock pigeon.
Both mutations nevertheless trigger similar biochemical processes in
the respective species and thus fulfil similar functions (Vickrey et
al., 2015, p. 2659ff.). Both traits are recessive in their
respective environment, but interact like alleles at the illustrated
intersection, so that the trait shows up in the hybrid. For the
different kinds of feather crests in different domestic pigeon
breeds, the research group had come to the conclusion in previous
studies that a mutation probably occurred a very long time ago. This
would have spread through crossbreeding to the different breeds
(Shapiro et al. 2013), for which there have been many references in
the domestic pigeon literature, at least for the last millennium.
The question remains, however, whether there is a bias for mutations
in the relevant genome area among the pigeon-like due to their
common origin and whether something like a common development
program exists for them, here for the hood. This is the subject of a
side note of the 2015 study (p. 2661f.) and could also apply
analogously to other phenomena, such as checks. That would call into
question the criterion of the D-statistic for the determination of
introgressions, applied to very narrow genome areas. This could also
explain the strikingly high values of the D-statistics here. The
mentioned possibility does not seem unique in the animal world.as
was shown by extensive studies on cichlids. The genetic environment
of diverging species could remain similar in certain areas without
showing a certain characteristic. However, it could be activated in
successor species in parallel by selective triggering mutations and
express a characteristic without introgression. For example, for
cichlids it is assumed that parallel evolutions take place quickly
and that certain features are fixed independently of one another in
different populations (Urban et al. 2020, p. 466).
Interesting from the point of view of gaining more experience about
similar phenomena and possible similar chains of effects, also the
relationship of C. livia to the cliff pigeon mentioned above. The
characteristic of the mirror tail, an analogy to checks, is found in
both domestic and wild pigeons. The lightening is not only evident
in Bern mirror tails, but also in other domestic pigeon breeds.
Thus, in the picture with a Frosty variant and a Turkish Takla
Tumbler. Molecular genetic studies specifically on the mirror tail
and possible parallels between domestic pigeons and rock pigeons do
not seem to be available. Also, whether it is genetically identical
in appearance in Bern mirror tails and other breeds has not yet been
investigated.
  
Fig. 14: Lightening in front of the tail band of domestic pigeons
and the cliff pigeon (Genetik der Taubenfärbungen 2015, Pigeon
Genetics 2012, p. 158
Further investigations into phenomena that occur in very similar
ways in the domesticated rock pigeon and Barbary pigeon could also
expand our knowledge of the underlying mechanism
Summary
One of the certainties that one believes to have taken away from
reading Darwin's book is that the domestic pigeon descends solely
from the rock pigeon. Molecular genetic studies that come to the
conclusion that the pigeons in the check patter that is widespread
today are supposed to carry the genetic makeup of another species,
the checkered C. guina, are surprising. Some indications are given
here that make this seem unlikely. It is the different distribution
areas of the two species and the problems of getting fertile hybrids
from crossing the species. In addition, there is the estimated time
in which an introgression should have taken place after the
calculations, and reports of observations that suggest a mutative
appearance of the check pattern, among other changes, in
domesticated or semi-wild pigeons instead of an introduction by
hybridization. The D statistical values of the molecular genetic
analysis, which indicate introgression, are not shown genome wide
but selectively in the 'candidate region' of the genome in which the
pattern traits are located. There may be other explanations for
this, such as mutations that repeat independently of each other.
These could be favored by a similar genetic environment inherited
from a common ancestor as still was supposed in other species. Thus,
it could be instructive to study with the same methodology, and also
a similar narrow sequence, relations between the domesticated
Barbary pigeon (Streptopelia 'risoria') in their recessive white,
albino and silky varieties and C. livia.
Literature:
Albin, Eleazar, Natural History of Birds.
Illustrated With a
Hundred and one Copper Plates, Engraven from the Life, Published by
the Author and carefully colour’d by his Daughter and Himself, from
the Originals, drawn from the live Birds, Vol III London MDCCXXXVIII
(1738)
Bechstein, Johann Matthäus, Gemeinnützige Naturgeschichte
Deutschlands nach allen drey Reichen, 4. Band Leipzig 1795
Bechstein, Johann Matthäus, Gemeinnützige Naturgeschichte
Deutschlands nach allen drey Reichen. Ein Handbuch zur deutlichern
und vollständigern Selbstbelehrung besonders für Forstmänner,
Jugendlehrern und Oekonomen, Dritter Band, Mit Kupfern, Zweite
vermehrte und verbesserte Auflage, Leipzig 1807
Bruders, R. u.a., (2020) A copy
number variant is associated with a spectrum of pigmentation
patterns in the rock pigeon (Columba livia). PLOS Genetics
16(5):1008274.
https://doi.org/10.1371/journal.pgen.1008274
Darwin, C. R. 1875. The variation of animals and plants under
domestication. London, John Murray. 2d edition. Volume 1
Dixon, E.S., The Dovecote and the Aviary, London 1851
Durand, Eric et al., Testing for Ancient Admixture between Closely
Related Populations, Mol.
Biol. Evol. 28(8):2239-2252, 2011
Fennelt, Dietmar, Forum Flügelvieh.de – Über Kreuzungen von
Wildtaubenarten http://forum.fluegelvieh.de/showthread.php?tid=238
Friedrich II, De arte
venandi cum avibus, Vatikan,
Biblioteca Apostolica Vaticana, Pal. lat. 1071 Friedrich <II.,
Heiliges Römisches Reich, Kaiser> Heidelberger historische Bestände
digital. Über die Kunst mit Vögeln zu jagen — Süditalien, 1258-1266;
Das Falkenbuch Kaiser Friedrich II. Nach der Prachthandschrift in
der Vatikanischen Bibliothek. Einführung und Erläuterungen von Carl
Arnold Willemsen, Harenberg.
Dortmund 1980
Goodwin, Derek, Pigeons and Doves of the World, British Museum
(Natural History), 2nd edition London 1970
Gray, Annie P., Birds Hybrids, A Check-List with Bibliographie,
Bucks, England 1958
Haag-Wackernagel, Daniel, Die Taube. Vom heiligen Vogel der
Liebesgöttin zur Straßentaube, Basel 1998
Kinzelbach, Ragnar K. und Jochen Hölzinger (Hrsg.), Markus zum Lamm,
Die Vogelbilder aus dem Thesaurum Picturarum, Ulmer: Stuttgart 2000
Miller, W.J. und J.R. Demro, Allelism of Crested Traits in Columba
Livia and Streptopelia Risoria, Iowa State Pigeon Assoc Bull. 2011,
S. 6-7
Raethel, H.-S., Wildtauben. Exotische Ziervögel, Ulmer: Stuttgart
1980
Sell, Axel, Critical Issues in Pigeon Breeding. What we know and
what we believe to know. Anecdotal, Entertaining, and Educational
comments on open questions Part 1, Achim 2020.
Sell, Axel, Genetik der Taubenfärbungen, Achim 2015
Sell, Axel, Pigeon Genetics. Applied Genetics in the Domestic Pigeon
Sell, Axel, Tauben. Züchten mit System, Reutlingen 1995
Sell, Axel, Taubenrassen. Entstehung, Herkunft, Verwandtschaften.
Faszination Tauben durch die Jahrhunderte, Achim 2009
Shapiro, Michael D., Genomic diversity and evolution of the head
crest in the rock pigeon, Science 2013 Mar 1, 339(6123): 1063-1067
Soares, André E.R. et al., Complete mitochondrial genomes of living
and extinct pigeons revise the timing of the columbiform radiation,
BMC Evolutionary Biology 2016
Stauber, Karl, Von Berner Spiegelschwänzen und spiegelschwänzigen
Klippentauben, Geflügel-Börse 8/2004, S. 14-16
Urban, Sabine, et al., Different Sources of Allelic Variation Drove
Repeated Color Pattern Divergence in Cichlid Fishes (University of
Konstanz), Mol. Biol. Evol. 38(2): 465-477 Advance Access
publication September 17, 2020
Vickrey, A. et al., Convergent Evolution of Head Crests in Two
Domesticated Columbids Is Associated with Different Missense
Mutations in EphB2, Mol. Biol. Evol. 2015
Vickrey, Anna I, E.T. Domyan u.a., Convergent Evolution of Head
Crests in Two Domesticated Columbids Is Associated with Different
Missense Mutations in EphB2, Oxford Journals. Molecular Biology and
Evolution 2015 June 23
Vickrey, Anna I. et al., Introgression of regulatory alleles and a
missense coding mutation drive plumage pattern diversity in the rock
pigeon, eLifesciences.org 2018
A.S.
|